The Flometrics Pressure Pulse Generator & Attenuation Testing Device (PPG-601A) is an all-in-one system for testing frequency response and attenuation of transducers, catheters and other pressure-sensing devices. The system was developed using Flometrics’ extensive knowledge of fluid mechanics and years of experience in the engineering services field. This system is in use by several of the world’s top medical device manufacturers.
Pressure and frequency capabilities can be custom-tailored for the required application. Typical specifications are listed below.
- Pressures from 0 to 200 torr
- Frequency up to 1khz
- Waveforms: Sine, Square, Triangle, Custom

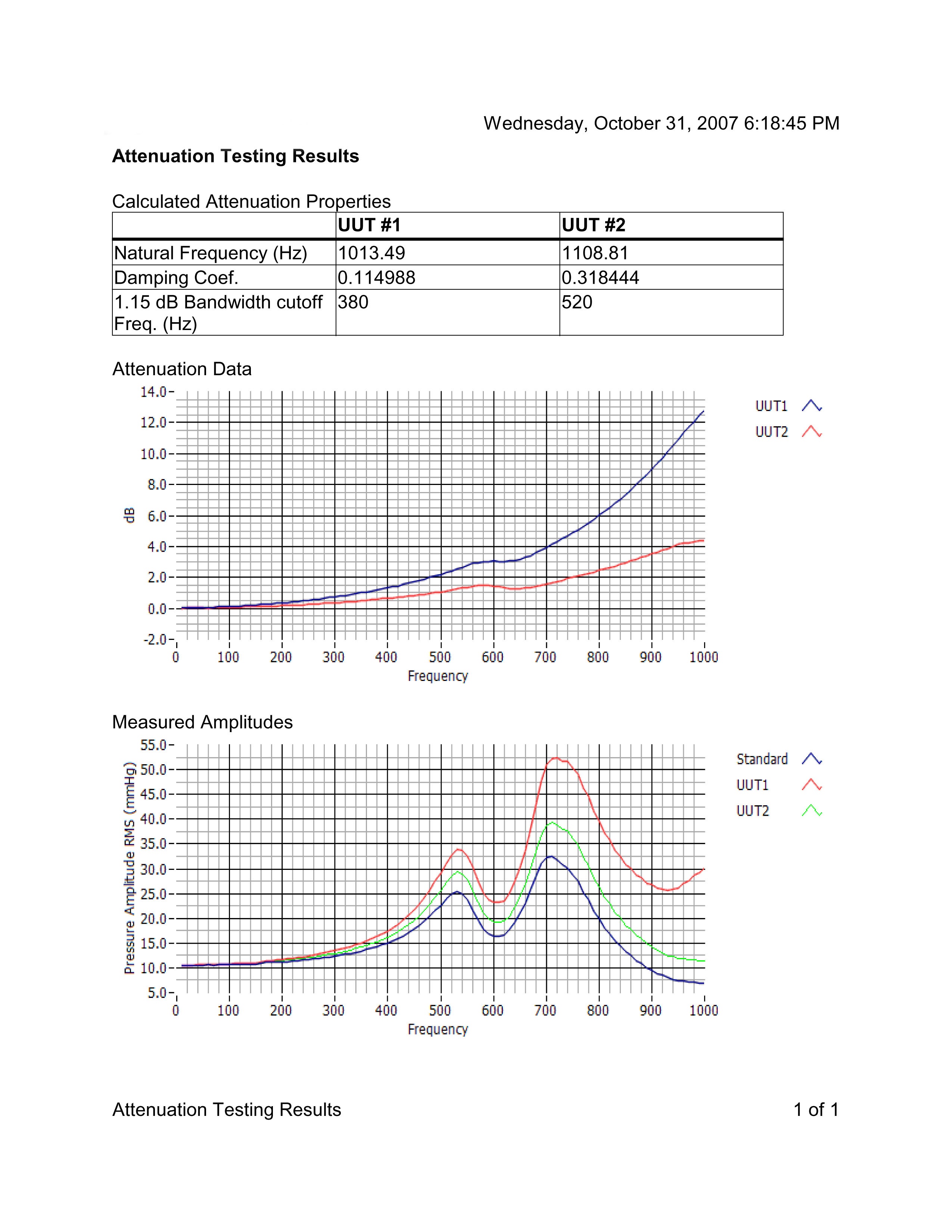
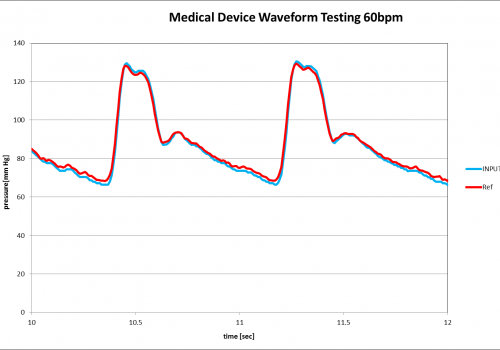
Medical Device testing results using the PPG-601A to output pressure vs time data
PPG-601A Configurations
The PPG-601A can be used in a variety of system configurations. Such systems can be simple as using only a function generator to output a pressure signal or a customized software made pressure signal. The software package offers the ability run Sine, Square and Triangle waveforms, plot of attenuation vs. frequency for each sensor tested with automatic FFT analysis, and custom waveform such as mimicking the human heart. All data will be saved in both detailed via spread sheet and summarized via PDF output. The pressure pulse generator unit is also available separately for customers who do not require automatic control and measurement. When used in this way, the device can be a substitute for the hard-to-find Biotek 601A.



Featured Set-Up: Test Catheters
The PPG-601A can be customized to your needs. Don’t know what to ask for? Our engineers are here to help. Below is an example of the PPG-601A setup to test catheters.